In June of 2024, the United States (US) Food and Drug Administration (FDA) approved Cepheid’s GeneXpert (XpertⓇHCV) for Point of Care (POC) hepatitis C (HCV) diagnostic testing, the first of its kind for HCV in the US. The test is CLIA (Clinical Laboratory Improvement Amendment) waived, meaning the instrument is simple to use and with low risk for error. CLIA-waived tests such as the XpertⓇHCV allows non-medical settings and/or non-medical personnel to perform the diagnostic test onsite with a fingerstick and deliver an HCV diagnosis within an hour.
Implementation in New York State
To support New York State (NYS) in its goal to eliminate hepatitis C, the Bureau of Hepatitis Health Care and Epidemiology, in collaboration with the Office of Drug User Health within the NYS Department of Health (NYSDOH), plans to provide funding and technical assistance to 12 Drug User Health Hubs statewide to purchase and implement the XpertⓇHCV POC test at their locations. These Health Hubs provide harm and risk reduction strategies and medication for opioid use disorder to people who use drugs and who are most at risk for HCV infection. An additional nine NYSDOH funded HCV Care and Treatment Providers will also receive funding to support the purchase of the XpertⓇHCV.
Advantages of Point of Care Diagnostic Testing
The current algorithm for HCV testing is a two-step process. First, an antibody test is conducted as a screening test, followed by an HCV RNA test for those clients with a reactive HCV antibody test. The RNA test is needed to confirm or rule out a diagnosis of an active hepatitis C infection. Prior to the FDA approval of the XpertⓇHCV, RNA testing involved a blood draw and sending the specimen to a lab for processing, which would require the patient to return for their test results. This can lead to patients not returning and not receiving a diagnosis, thereby not receiving treatment for hepatitis C.
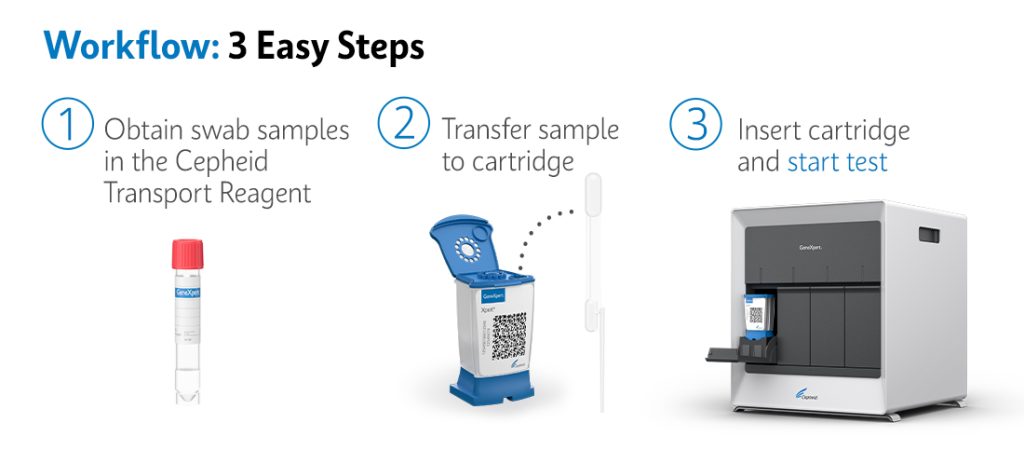
The XpertⓇHCV allows for non-medical personnel to collect, test, and give diagnostic results within 41-60 minutes after collection, potentially eliminating lost-to-follow-up outcomes. Instead of a blood draw that requires a phlebotomist, the XpertⓇHCV uses fingerstick blood. With a rapid diagnosis, HCV treatment initiation can begin as soon as same day of diagnosis.
Key Considerations
When considering the incorporation of the XpertⓇHCV for HCV diagnostic testing, several factors should be taken into account. The cost of the instrument, including software maintenance fees and the per-test cartridge cost, may be prohibitive for some organizations. Additionally, the test’s run-time of up to 60 minutes results in longer patient wait times, which could be a significant consideration for many patients. Furthermore, XpertⓇHCV is not currently FDA approved for use in pregnant individuals, persons under 22 years of age, or to assess for cure of HCV (Sustained Virologic Response). Despite all of these factors, the approval of the XpertⓇHCV is a major step towards timely HCV treatment initiation and HCV elimination.
