This HCV Dashboard Blog program spotlight features a guest contribution from Luke Grandis at the New York State Department of Health. Hepatitis C (HCV) remains a major public health issue in New York State, particularly among people who inject drugs (PWID), who continue to experience disproportionately high rates of infection. Injection drug use is the […]
Tag Archives: HCV Programs
In June of 2024, the United States (US) Food and Drug Administration (FDA) approved Cepheid’s GeneXpert (XpertⓇHCV) for Point of Care (POC) hepatitis C (HCV) diagnostic testing, the first of its kind for HCV in the US. The test is CLIA (Clinical Laboratory Improvement Amendment) waived, meaning the instrument is simple to use and with […]
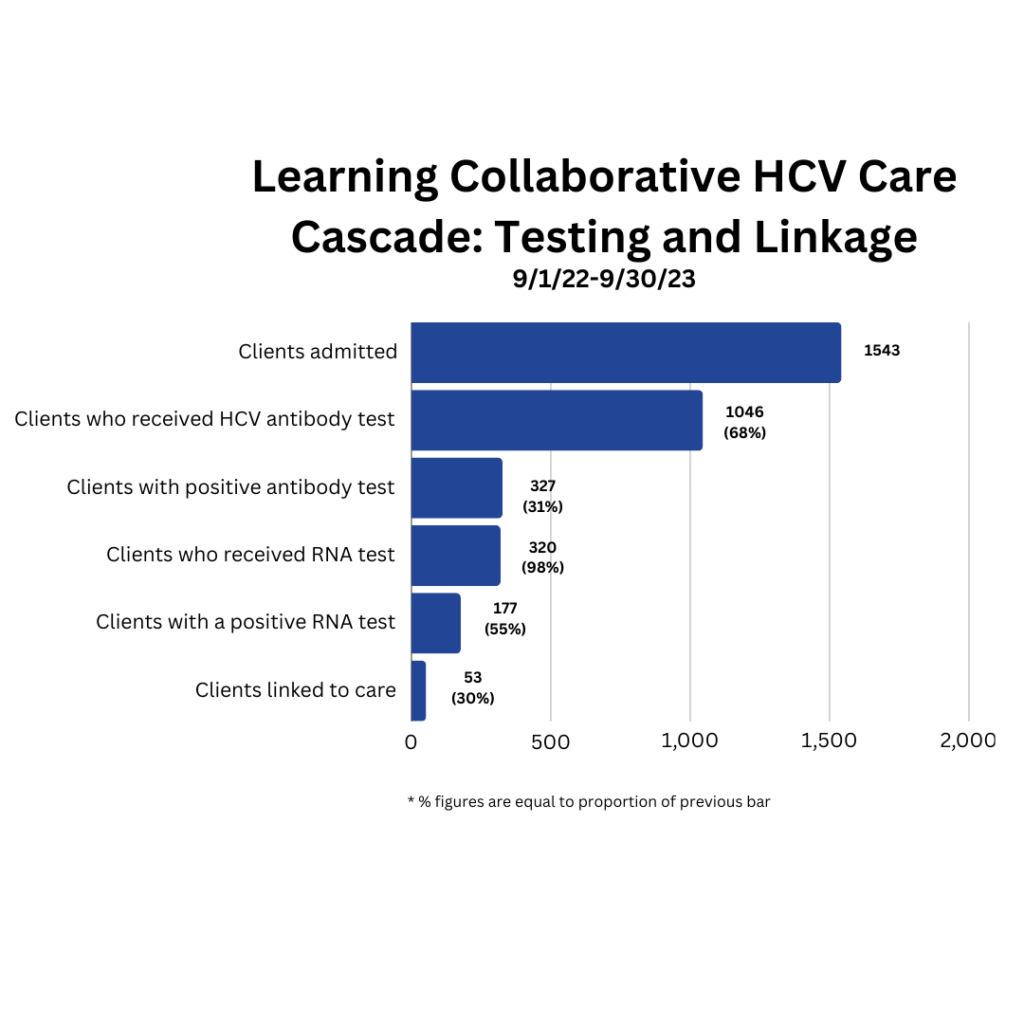
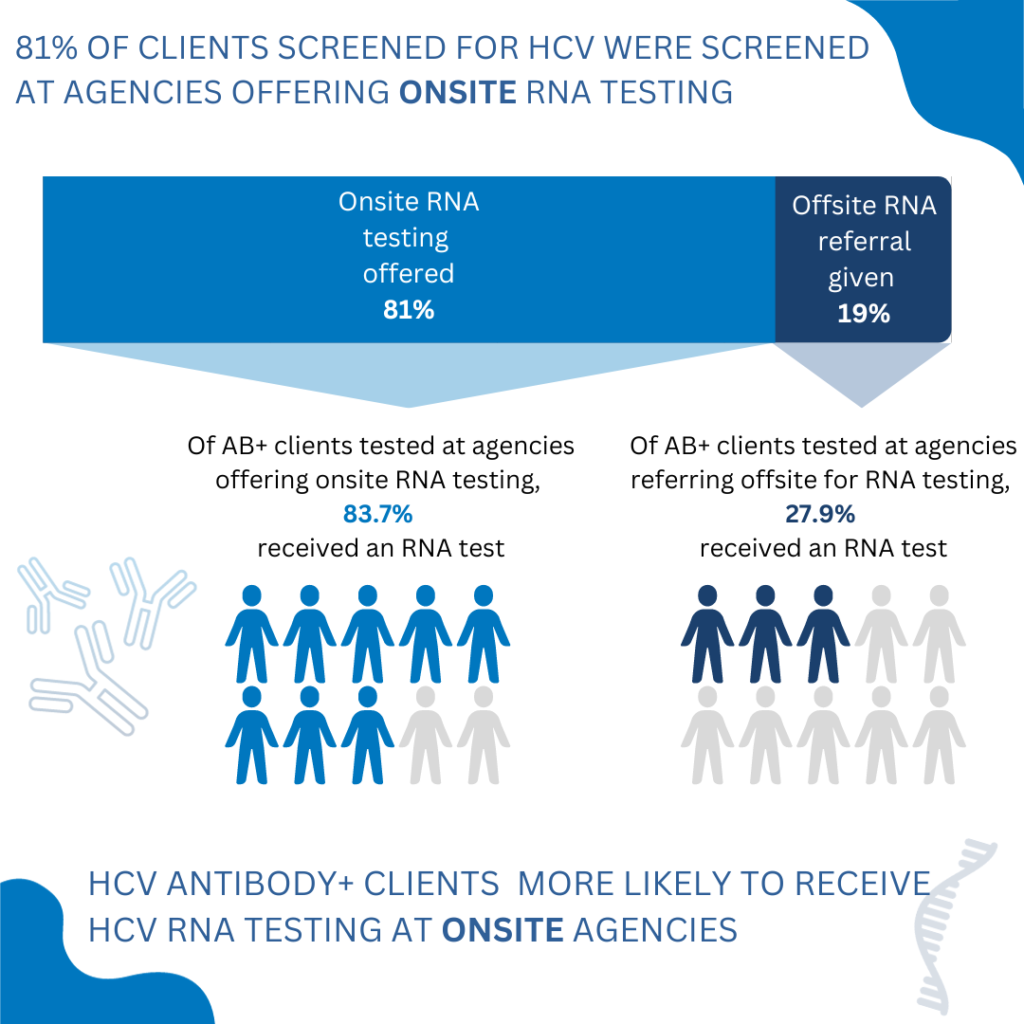
What is the Learning Collaborative? The New York State Hepatitis C Learning Collaborative for Substance Use Disorder Treatment Programs is a two-year initiative to help programs build capacity to provide onsite HCV testing and linkage to care. The New York State Department of Health (NYSDOH) launched the first cycle of the learning collaborative on April […]
Dear Partners in Elimination, We invite you to share your innovative and promising practices that support New York State’s hepatitis C elimination efforts. Selected submissions will be invited to present during the 2024 Annual NYS Hepatitis C Elimination Progress Report Meeting, to be held virtually on May 15th, 2024. Each presenter will provide a 10-minute oral presentation. This is your chance to highlight your […]
Since 2019, the New York State (NYS) Department of Health AIDS Institute has funded three “innovative models” to provide HCV care and treatment located in settings accessible to PWID, including co-location at a syringe services program/drug user health hub, via mobile van, onsite at drug treatment facilities, as well as using telehealth.,,
Several of the NYS HCV Elimination Plan’s recommendations focus on hepatitis C virus (HCV) testing including universal HCV screening among adults and pregnant people, as well as expanding point of care HCV testing into high-risk settings.